Sustained drug release
Maintained efficacy
Improved patient compliance
Reduced patient costs

Long-Acting Injectable for Chronic Diseases
80% of adults over the age of 65 have at least one chronic condition. These patients have to continuously take drugs. Since the majority of current treatments require daily intake of drugs, it is a hassle to keep such regimens.
The prefilled injectable platform technology, MEDI-DEPOT™ maintains sustained release of drugs once in the body. This can overcome the hassle of repeatedly taking drugs everyday as patients take just ONE injection every month.

The population is aging. According to the World Health Organization (WHO), many of those over 65 years old have at least one chronic condition such as arthritis, dementia, hypertension, and deafness. For these patients and their caretakers, adhering to current drug regimens is an issue as it is easy to forget or overdose.
Conventional drugs require daily, or at most weekly, intake. This is because pills (tablets) or patches have short-term efficacy. For drugs to have effects that can relieve symptoms of diseases, the drug concentration in the human body has to be within the therapeutic window. Treatments available today reach this window but is cleared out from the bodily system quite rapidly. Thus, the repeated dosing of drugs.
MEDIPOLYMER’s solution to this problem is MEDI-DEPOT™. Using conventional drugs with proven therapeutic effects, our technology prolongs its efficacy to weeks and even months with just ONE injection. This is possible as the hydrogel formulation, once injected, becomes a depot and steadily releases the loaded-drug into the body.
MEDI-DEPOT™ not only overcomes the hassle of daily/weekly drug regimens but further increases patient compliance and reduces treatment costs. With our continuous efforts in developing the prefilled injectable platform, possibilities to enhance current treatment options for various geriatric and intractable disease patients are endless.
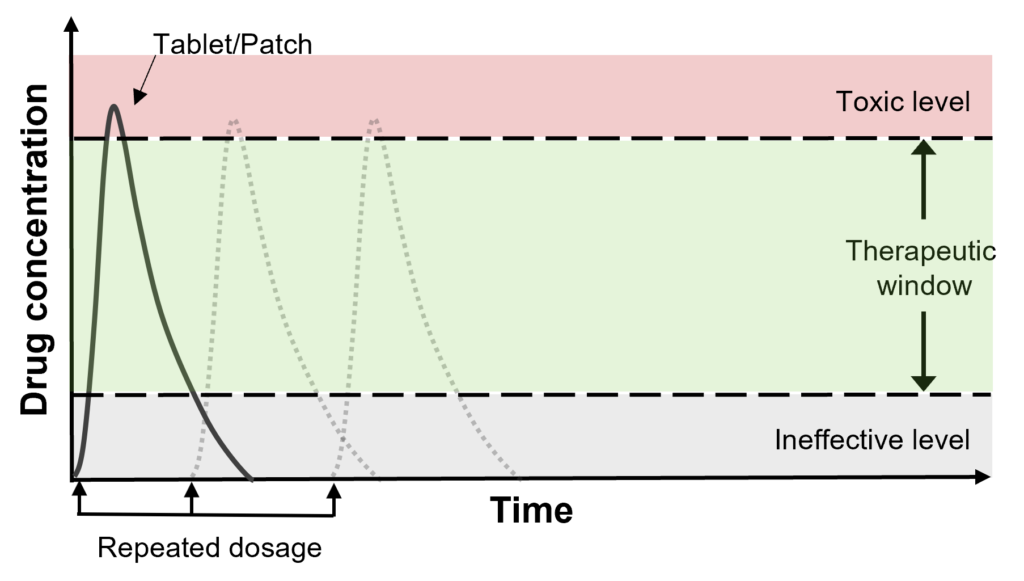
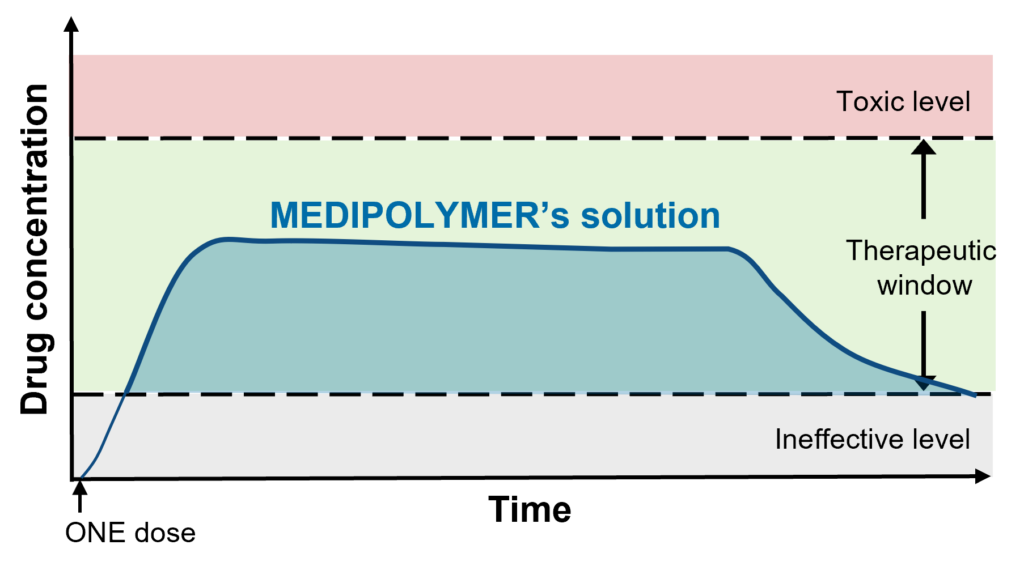
Preliminary in vivo results confirmed 1 month drug release within therapeutic window.
By modifying the formulation, sustained release was observed up to 3 months in vivo.